Electrolysisnoun
(chemistry) the chemical change produced by passing an electric current through a conducting solution or a molten salt.
Electrolysisnoun
The destruction of hair roots by means of an electric current.
Electrolysisnoun
The act or process of chemical decomposition, by the action of electricity; as, the electrolysis of silver or nickel for plating; the electrolysis of water.
Electrolysisnoun
lysis of a bond produced by the passage of an electric current
Electrolysisnoun
removing superfluous or unwanted hair by passing an electric current through the hair root
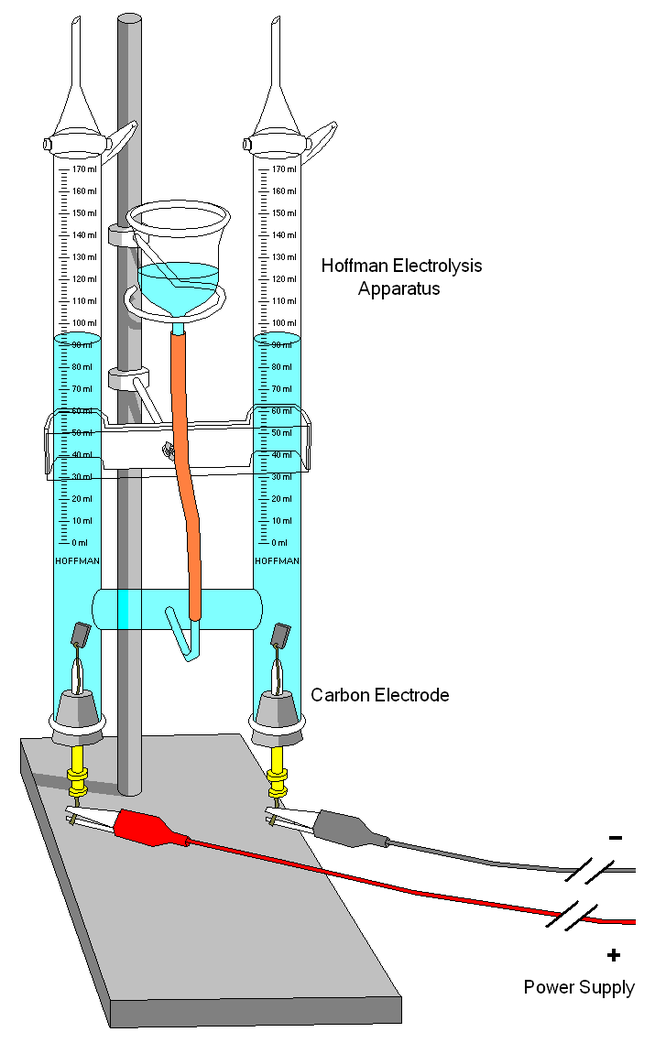
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell.
Electroplatingnoun
A process of coating the surfaces of a metal object with a layer of a different metal through electrochemical means, usually to exploit different properties of the materials.
Electroplatingnoun
The art or process of depositing a coating (commonly) of silver, gold, or nickel on an inferior metal, by means of an electric current. The metal to be deposited on an article is usually used as the anode and the article to be plated as the cathode, in an electrolyte solution in which the plating metal is the cation. The process is conducted in a tank called an electroplating bath, which holds the electrolyte solution.
Electroplating
Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode (positive electrode) is usually either a block of that metal, or of some inert conductive material.